Core Concepts
Temperature
A measure of average kinetic energy of particles.

Heat
Energy transferred due to temperature difference.
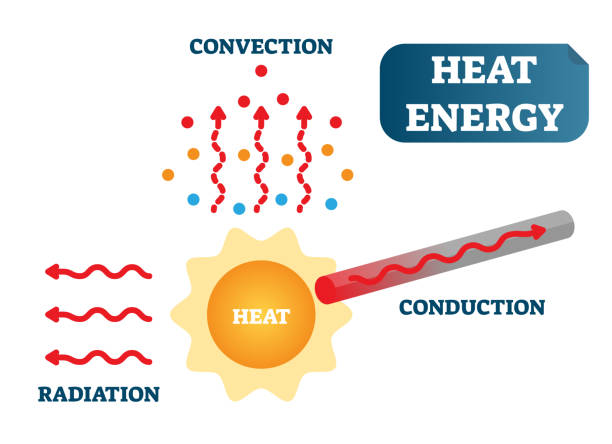
Internal Energy
Total kinetic and potential energy of particles.
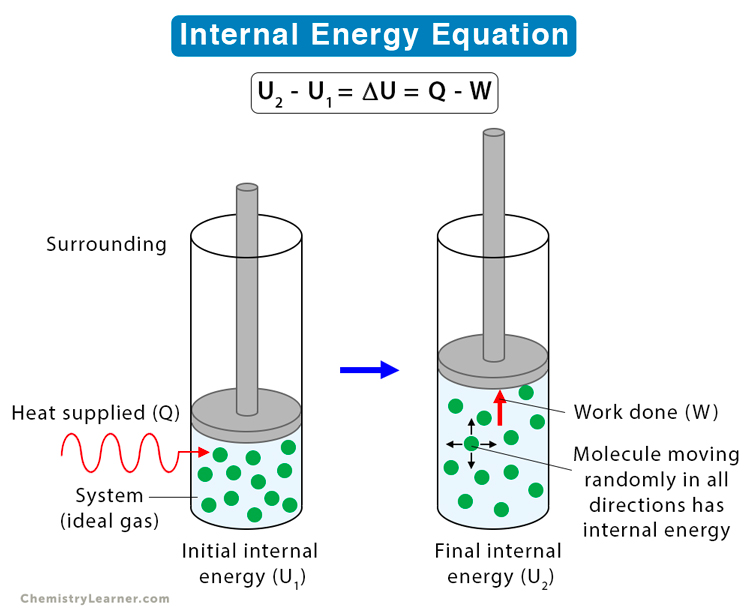
Thermodynamics is the branch of physics that deals with heat, temperature, and their relation to energy and work. It involves laws that describe how energy moves and changes in a system.
A measure of average kinetic energy of particles.

Energy transferred due to temperature difference.

Total kinetic and potential energy of particles.

Heat transfer
First Law
Boyle’s Law
PV = nRT

Solution: Q = mcΔT = 2 × 4200 × 20 = 168,000 J
Solution: ΔU = Q - W = 500 - 200 = 300 J
Solution: V₂ = P₁V₁ / P₂